Introduction of Nucleus
The study of nuclei involves understanding the structure and behaviour of atomic nuclei, which are at the heart of an atom. The nucleus contains protons and neutrons, bound together by a force called the nuclear force. ‘
Nuclear physics explores phenomena like radioactivity, nuclear reactions, and the energy released in nuclear processes. It also plays a crucial role in energy generation through nuclear fission and fusion.
Atomic Masses and Composition of Nucleus
- Atomic Mass Unit (amu): The mass of atoms and nuclei is measured in atomic mass units. One atomic mass unit is defined as 1\12 the mass of a carbon-12 atom.
- Protons and Neutrons: The nucleus consists of protons (positively charged) and neutrons (neutral). The total number of protons is the atomic number (Z), and the total number of nucleons (protons + neutrons) is the mass number (A). For example, in a carbon-12 atom, Z=6 (6 protons) and A=12(6 protons + 6 neutrons).
- Mass of Nucleus: The mass of the nucleus is almost entirely due to protons and neutrons. The mass of an individual proton or neutron is approximately 1 amu.
Size of the Nucleus
The size of a nucleus is very small compared to the size of the entire atom. The radius of the nucleus R is given by the relation:
Where ![]() m is a constant, and A is the mass number.
m is a constant, and A is the mass number.
The nucleus is about 10 –14m in radius, much smaller than the atom’s size, which is about 10 –10m.
Nuclear Force
Nuclear Force is the force that holds protons and neutrons together in the nucleus is called the nuclear force. It is a short-range, attractive force that is much stronger than the electrostatic force between protons but operates only over distances of about 10–15m.
Characteristics of Nuclear Force:
- Short-Range: The nuclear force is attractive at very short distances (around m) but becomes repulsive at even shorter distances.
- Isotropic: It acts equally in all directions between nucleons (protons and neutrons).
- Independence from Charge: Unlike the electrostatic force, the nuclear force does not depend on the electric charge, so it acts between both protons and neutrons.
Types of Nuclear Forces
Types of Nuclear Forces:
- Strong nuclear force: This force is responsible for holding protons and neutrons together in an atomic nucleus.It is the strongest of all fundamental forces, about 130 times stronger than electromagnetic forces. It helps overcome the coulomb’s barrier, i.e. the barrier posed by the electromagnetic forces.
- Weak nuclear force: This force is responsible for the radioactive decay of some nuclei.It also initiates the process of nuclear fusion. The weak force changes the “flavour” of a quark, changing it from an up quark to a down quark or vice versa.
| Four Fundamental forces of nature | ||||||||||
|
Radioactivity
Radioactivity is the spontaneous emission of radiation from the nucleus of certain unstable atoms. This occurs because the nucleus of these atoms is unstable, and to reach a more stable configuration, they emit particles or electromagnetic radiation.
Types of Radiation:
- Alpha (α) Radiation: Emission of an alpha particle (two protons and two neutrons) from the nucleus.
- Beta (β) Radiation: Emission of a beta particle, which is an electron, due to the decay of a neutron in the nucleus.
- Gamma (γ) Radiation: High-energy photons emitted from the nucleus after other types of radiation occur, typically without changing the number of nucleons.
Radioactive Decay
The rate of radioactive decay is the speed at which a radioactive substance decays, and is a unique property of each radioactive isotope. It can be characterized by the following parameters:
- half-life (T): The time it takes for half of a radioactive substance to decay. Half-lives can range from seconds to millions of years.
- Decay constant: The rate constant, represented by the Greek letter lambda (λ), is measured in units of time.
Radioactive decay is the random process where an unstable nucleus loses energy by emitting radiation. The rate of decay is characterized by the which is the time taken for half the atoms in a sample to decay.
Carbon Dating
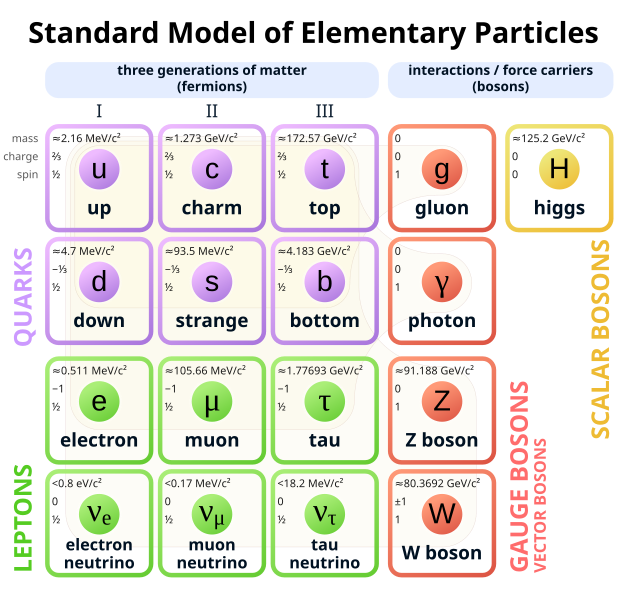
Fundamental Particles in Nature
The Fundamental particles of nature or the elementary particles of nature are the subatomic particles that are not composed of other particles.
All elementary particles are either bosons or fermions. These classes are distinguished by their quantum statistics: fermions obey Fermi–Dirac statistics and bosons obey Bose-Einstein statistics.
Their spin is differentiated via the spin-statistics theorem: it is half-integer for fermions and integer for bosons.
Spin Quantum Number
Spin (s) is a quantum number that describes the intrinsic angular momentum of any particle. It has the same value for all particles of the same type.
- Those particles with integer spin are bosons. For example Photon.
- Half spin for all fermions.
- Scalar Bosons are those particles that have no spin. Till now only one such particle fundamental particle, Higgs Boson, is known.
The component of the spin along a specified axis is given by the spin magnetic quantum number. Atomic nuclei also have spins composed of the spin of the independent particles.
Detection of spin
When the spectral lines of Hydrogen (Hydrogen spectrum) are examined at a very high resolution, they are found closely spaced doublets. The direct observation of the electron’s intrinsic angular momentum was achieved in the Stern-Gerlach Experiment.
Stern-Gerlach Experiment: When silver is atomised in a vacuum- electric furnace and guided into a flat beam and directed through a uniform magnetic beam, it should make a band according to classical physics. But it made two bands, confirming two quantum states.
Quarks
Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of’ atomic nuclei.
Flavour of Quarks
Quarks are elementary particles that come in six different flavors, or types: up, down, charm, strange, top, and bottom. Each flavour has unique properties, such as mass, charge, and interaction characteristics.
- Up quarks: Have a charge of +2/3
- Down quarks: Have a charge of -1/3
The “strong nuclear force” holds together the nucleus of an atom by counteracting the tendency of the positively-charged protons to repel each other.
Hadrons
Protons and neutrons are made of quarks, which are fast-moving points of energy:
- Protons: Contain two up quarks and one down quark, giving it a net positive charge.
- Neutrons: Contain one up quark and two down quarks, making it neutral
These triplet of Quarks are stabilised by the particles called Gluons, which are massless Bosons.
| Large Hadron Collider |
Leptons
Leptons are those elementary particles that have half-integer spin, and that are not influenced by the strong nuclear force (also known as strong interactions). Two classes of leptons exist:
- Charged Leptons: These include electrons, Muons and Tau particles.
- Neutrinos or the Neutral Leptons.
Electron
It is a sub-atomic particle with a negative charge. It is generally denoted as e – but also as b – in nuclear reactions (beta decay). Its mass is approximately of the mass of the Proton. Electrons have the least mass of all three charged leptons.
These were discovered by JJ Thompson, through his experiments with the cathode ray tube.
Neutrino
It is an elementary particle, a lepton that interacts via weak interaction and gravity.
- It is called a neutrino because it is electrically neutral.
- Its rest mass is so small that it was long thought to be zero.
Its gravitational interaction is extremely weak and neither does it participate in the electromagnetic interactions nor in strong force, and the weak nuclear force is very weak.
Weak interaction creates neutrinos of three leptonic flavours.
- Electron neutrino
- Muon neutrino
- Tau neutrino.
Since they rarely interact, it is very difficult to observe neutrinos.
|
India-based Neutrino Observatory (INO) |
INO is a particle physics research project. It is located in a 1200 m deep cave under the INO peak in the Bodi West Hills of the Theni district of Tamil Nadu (Theni hills).
|