Biotechnology
Biotechnology refers to the technology sector that utilises biological systems, living organisms or enzymes or parts from the organisms to produce products and processes useful to humans. It essentially involves the integration of natural sciences and engineering sciences to achieve the application of organisms and parts thereof for products and services.
Defining Biotechnology
In today’s world: The term Bio-Technology is used in a restricted sense today, to refer to such processes that use genetically modified organisms to achieve the same on a larger scale.
European Federation of Biotechnology (EFB) definition: ‘The integration of natural science and organisms, cells, parts thereof, and molecular analogues for products and services’.
Principles of Biotechnology:
- Genetic engineering: Techniques to alter the chemistry of genetic material (DNA and RNA), to introduce these into host organisms and thus change the phenotype of the host organism.
- The techniques of genetic engineering which include the creation of recombinant DNA, use of gene cloning and gene transfer, overcome the limitation of sexual reproduction (which creates multiple hybrids).
- Genetic engineering allows us to isolate and introduce only one or a set of desirable genes without introducing undesirable genes into the target organism.
- Maintenance of sterile ambience in chemical engineering processes to enable the growth of only the desired microbe/eukaryotic cell in large quantities for the manufacture of biotechnological products like antibiotics, vaccines, enzymes, etc.
Important Definitions
Let’s start this chapter with a few important terms
- Gene: It is a sequence of nucleotides in DNA or RNA.
- Genotype: Pattern of genes in an organism’s DNA that is responsible for a particular trait.
- Phenotype: It refers to the observable physical properties of an organism. These include the organism’s appearance, development, and behaviour.
- Germline: Inherited genetic material that comes from the eggs or sperm and is passed on to offspring.
Cells:
- Stem cells: Undifferentiated cells that can turn into any specific cells, as the body needs them. These are found in small numbers in most adult tissues, such as bone marrow or fat.
Compared with embryonic stem cells, adult stem cells have a more limited ability to give rise to various cells of the body.
Stem cells can be of 3 Types:
- Totipotent cells can form all the cell types in a body + extraembryonic, or placental, cells. Embryonic cells within the first couple of cell divisions after fertilization are the only cells that are totipotent.
- Pluripotent cells can give rise to all of the cell types that make up the body; embryonic stem cells are considered pluripotent.
- Multipotent cells can develop into more than one cell type, but are more limited than pluripotent cells; adult stem cells and cord blood stem cells are considered multipotent.
- Somatic cells: Cells that are not involved in reproduction. Example, Muscle, skin, bone cells etc.
- Germ cells: They are cells that create reproductive cells called gametes. They are found only in the reproductive glands (ovaries in females and testes in males.).
Genome:
A Genome is the entire DNA, or sequence of genes, in a cell. A genome is divided into chromosomes, which contain genes, which are made of DNA.
- Human cells have 23 pairs of Chromosomes – 46 in total. Visible only when a cell is about to divide. All the other times they exist as Chromatin Material.
- Each genome has approximately 3.2 billion DNA base pairs.
Now let’s see the key technologies involved in the Biotech industry and their applications.
Genome mapping/sequencing:
Deciphering the exact order of base pairs in an individual by figuring out the order of DNA nucleotides, or bases, in a genome, i.e. the order of Adenine, Cytosine, Guanine, and Thymine that make up an organism’s DNA.
Two methods are commonly used for Genome sequencing:
- Sanger method (Whole Genome Sequencing), Maxam & Gilbert Method, Chain termination method, Pyrosequencing, clone by clone sequencing.
- Next Generation Sequencing(NGS) technologies: they provide massively parallel analysis and extremely high throughput from multiple samples at a much-reduced cost.
- It helps in generating large-scale genomic data critical for diagnosis and therapy.
- NGS facility: recently launched in Centre for Cellular and Molecular Biology, Hyderabad.
- Gene sequencing facility opened in 2020 in LNJP Hospital.
Genome Sequencing projects
Several Genome Sequencing projects have been running around the world.
Mapping of Species:
- Earth BioGenome Project: A UK-led project to code tens of thousands of vertebrates.
- Chinese Project: to Sequence 10,000 plants.
- Global Ant Genomes Alliance: 200 Ant Genomes.
Human Genome Mapping
Human Genome Project (1990-2003)
It was an international scientific research project to determine the base pairs that make up human DNA and to identify and map all of the genes of the human genome from both a physical and a functional standpoint.
- It remains the world’s largest collaborative biological project.
- Planning started after the idea was picked up in 1984 by the US government.
- Limitations – Narrow sample: Existing global genetic studies of the Human Genome Project are based mainly on Caucasian; urban middle-class samples (95%), which are not considered representative of all humans.
India’s Genome Mapping Programs
- “IndiGen” programme: CSIR has in the past concluded a six-month exercise of conducting a “whole-genome sequence” of 1,008 Indians under the “IndiGen” programme.
- Result: 32% of genetic variations in Indian genome sequences are unique.
- Genome India Project: cleared by the Department of Biotechnology (M/o ST)
- Estimated Budget worth of Rs 238 crores. [Promised in 2020 Budget]
- Aim: to map India’s genetic landscape to create a comprehensive database.
- Participating Institutes: It involves 20 leading institutions including
- Centre for Brain Research, an autonomous institute of IISc, Bengaluru- nodal point.
- Few IITs will also participate.
- The first stage of the project will look at samples of 10,000 persons from all over the country to form a grid that will enable the development of a Reference Genome.
- MANAV: Human Atlas initiative: By Dept. of Biotechnology (DBT): Mapping every single tissue of the Human body, to study the role of each tissue.
- Participating institutes: National Centre for Cell Science (NCCS) and IISER, Pune.
- Persistent Systems Limited has co-funded it (with DBT) & is developing the platform.
- The project can be signed up by students who are in their final year of graduation and above. Even participants having a science background but not necessarily involved in active scientific research can be part of this network.
- It will be helpful in Physiological and molecular mapping, Drug discovery, customising and personalising medicine, Skill development of the student community and Future research.
- It will capture human physiology in two stages – in a normal stage & in a disease stage.
- Such a database on individual tissues will help trace the causes of a disease, understand specific pathways etc.
- UMMID (Unique Methods of Management and Treatment of Inherited Disorders): Under the Department of Biotechnology(DBT), would also produce skilled clinicians in Human Genetics.
- Participating institutes: National Centre for Cell Science (NCCS) and IISER, Pune.
Advantages of Human Genome Mapping
- Predictive and Preventive Healthcare: It will help to understand the type & nature of diseases and traits that comprise the diverse Indian population and will help to develop precision medicine.
- Rare disease: It will help in the faster and more efficient diagnosis of rare genetic diseases.
- Scientific understanding of evolution both from a biological and sociological (migration patterns, rituals, etc.) point of view.
- Develop indigenous capacity: to generate, maintain, analyze, utilize and communicate large-scale genome data, in a scalable manner.
- Used as a tool in Forensic Science.
Human Microbiome:
The collective genome of all micro-organisms contained within the human body, residing inside tissues and bio-fluids. It includes bacteria, archaea, fungi, protists and viruses. It makes up around 2% of the body mass of the adult and is 1.3 times more than human cells in numbers.
- Most of them have either commensal (coexist without harming humans) or mutualistic (each benefit from the other) relations.
- It is shaped by factors such as genetics, dietary habits, age, geographic location and ethnicity.
- Role of Microbiology in Human Physiology:
- Metabolism of otherwise complex indigestible carbohydrates and fats.
- Production of essential vitamins
- Maintaining immune systems
- Acting as a first line of defence against pathogens.
- Determines how one responds to a particular drug treatment
Human Microbiome Project
It is a research initiative launched in 2007 by the US’s National Institute of Health with the mission to generate the resources and expertise needed to characterize the human microbiome and analyze its role in health and disease.
Methodologies used in HMP are:
- Metagenomics is a sequence-based approach that allows the genetic material from the complete collection of microbes to be analyzed in their natural environment without needing to cultivate the microorganisms.
- Whole Genome Sequencing (WGS) to provide a “deep” genetic perspective on aspects of a given microbial community, i.e. individual bacterial species.
Indian Human Microbiome Initiative
The Indian Human Microbiome Initiative is led by the National Centre for Microbial Resource (NCMR) – National Centre for Cell Science (NCCS).
- Aim: Identifying and characterizing human microbial fauna and elucidating their roles in health and diseases. The project will include a collection of saliva, stool and skin swabs of 20,000 Indians across various ethnic groups from different geographical regions.
- It is found that the Indian population, particularly tribals, have distinct gut microbiota and a lower prevalence of lifestyle diseases.
One Day One Genome Initiative
The ‘One Day One Genome’ initiative aims to showcase India’s vast microbial diversity and its vital role in the environmental, agricultural, and human health sectors. By sequencing and sharing bacterial genomes, it promotes public access, research, and innovation in these fields.
Cloning
Cloning refers to the production of an exact copy of a cell, any other living part, or a complete organism. Cloning is the process of producing individual organisms with identical or virtually identical DNA, either by natural or artificial means.
- In nature, some organisms produce clones through asexual reproduction.
- In the field of biotechnology, cloning is the process of creating cloned organisms (copies) of cells and DNA fragments.
First mammal Clone: Dolly, born on 5th July 1996. Performed by Ian Wilmut & his colleagues at Roslin Institute of Edinburgh, Scotland.
- A somatic cell was collected from the mammary gland of a female Finn Dorsett sheep. Simultaneously, an egg was obtained from a Scottish blackface ewe whose nucleus was taken.
- Since the nucleus from the egg of Scottish blackface was removed, Dolly didn’t show any character of Scottish blackface ewe & was a healthy clone of Finn Dorsett sheep & produced several offspring of her own through normal sexual means.
- Died on 14th Feb 2003 of lung disease.
- Several attempts have been made to produce cloned mammals since then but many die before or soon after birth; many a time found to be born with severe abnormalities.
Gene Editing
Gene Editing (Genome editing) is a group of technologies that give scientists the ability to change an organism’s DNA by adding, removing, or altering it at particular locations in the genome.
Transgene: A genetic material that is artificially introduced into the genome of another organism.
rDNA Technology
Recombinant DNA Technology (rDNA technology), also known as genetic engineering, is a process used to manipulate an organism’s DNA by combining genetic material from different sources to create new genetic combinations. It involves cutting, modifying, and recombining DNA from different organisms to achieve desired traits.
Three basic steps in genetically modifying an organism —
- Identification of DNA with desirable genes;
- Introduction of the identified DNA into the host;
- maintenance of introduced DNA in the host and transfer of the DNA to its progeny.
Stanley Cohen and Herbert Boyer Method (1972):
- Plasmid (Extra-Chromosomal DNA) can be used as a vector to deliver an alien piece of DNA into the host organism. The linking of the antibiotic resistance gene with the plasmid vector became possible with the enzyme DNA ligase, which acts on cut DNA molecules and joins their ends.
- The ability to multiply copies of antibiotic resistance genes in E. coli was called Cloning.
| Fact-File: Recreated Escherichia coli (E coli): the world’s first living organism that has a fully synthetic and radically altered DNA code by replacing the genes of E. coli bacteria with genomes synthesized in the lab. E-Coli has a relatively small DNA sequence. |
Steps involved in rDNA Technology:
- Isolation of DNA: DNA is extracted from the organism of interest (e.g., bacteria, plants, or animals). This step involves breaking open cells to release DNA and purifying it for further use.
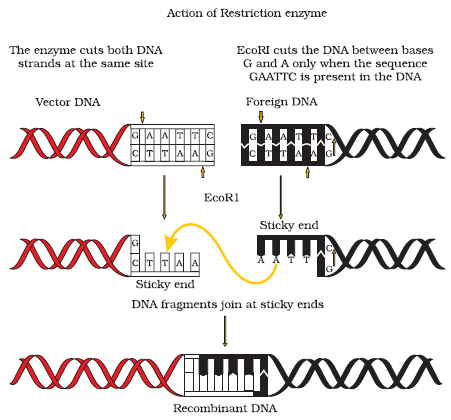
- Cutting the DNA: Restriction enzymes (also known as molecular scissors) are used to cut DNA at specific sequences. Restriction Enzymes called restriction endonuclease-Hind-II were first used in 1963. It always cuts DNA molecules at a particular point by recognizing a specific sequence of six base pairs, known as the recognition sequence.
Today we know more than 900 restriction enzymes that have been isolated from over 230 strains of bacteria each of which recognises different recognition sequences. Restriction enzymes belong to a larger class of enzymes called nucleases. These are of two kinds;
-
- Exonucleases remove nucleotides from the ends of the DNA,
- Endonucleases make cuts at specific positions within the DNA.
3. Joining the DNA: When cut by the same restriction enzyme, the resultant DNA fragments have the same kind of ‘sticky-ends’ and, these can be joined together (end-to-end) using DNA ligases. The cut DNA from one organism is combined with the DNA of another using an enzyme called DNA ligase, which seals the DNA fragments together to form a recombinant molecule.
- Cloning the Recombinant DNA: The recombinant DNA is inserted into a vector (such as a plasmid or a virus) that carries the foreign DNA into a host cell. The host cell, typically a bacterium (like coli), is then used to propagate the recombinant DNA.
- Selection and Screening: After the recombinant DNA has been introduced into the host cell, it is grown in a medium where only those cells containing the recombinant DNA will survive. The recombinant cells are then screened or tested to ensure they carry the desired genetic modification.
- Expression of Desired Traits: The host organism (often bacteria, yeast, or mammalian cells) is induced to express the foreign gene. This could result in the production of a desired protein, such as insulin, or the modification of an organism’s characteristics.
Applications of rDNA Technology:
- Medicine:
- Production of Insulin: rDNA technology is used to produce human insulin by inserting the insulin gene into bacteria or yeast.
- Gene Therapy: It involves replacing defective genes with healthy ones to treat genetic disorders.
- Vaccines: Recombinant vaccines, like the Hepatitis B vaccine, are made by inserting viral genes into host cells to produce antigens without using the actual virus.
- Agriculture:
- Genetically Modified Crops: rDNA technology is used to create crops with improved traits, such as pest resistance (Bt cotton) or increased nutritional content (Golden Rice).
- Transgenic Animals: Animals can be genetically modified for research or agricultural purposes, such as producing milk with human proteins.
- Industrial Biotechnology:
- Enzyme Production: rDNA technology is used to produce enzymes that are used in food processing, detergents, and biofuels.
- Bioremediation: Genetically engineered microorganisms can be used to clean up environmental pollutants, such as oil spills.
- Research:
- Gene Cloning: rDNA technology is used to clone specific genes for study, allowing scientists to investigate gene function and expression.
- Gene Mapping: The technology is used to map genomes and study genetic variations in organisms.
Ethical Considerations:
- Environmental Impact: The release of genetically modified organisms (GMOs) may have unintended ecological consequences.
- Health Concerns: There are concerns about the safety of genetically modified foods and organisms.
- Ethical Issues in Medicine: The use of gene editing, particularly in humans, raises ethical concerns about “designer babies” and genetic modification.
CRISPR
CRISPR stands for Clustered Regularly interspaced short Palindromic repeats. It was discovered in 2012 and held as the “Breakthrough of the Year” in 2015 by the U.S. journal Science.
- Mechanism: Bacteria have a mechanism of producing clustered regularly interspaced short palindromic repeats in their gene sequence (a memory to fight viruses) = Bacteria’s immune system.
- These special gene sequences are copied into RNA and loaded into a special protein/enzyme called Cas9 which can be used to edit DNA.
- CRISPR is a collection of DNA sequences that direct Cas9 where to cut and paste.
- It allows scientists to selectively edit genome parts and replace them with new DNA stretches.
Applications of CRISPR till Now:
- Human genome editing: Sichuan University, Chengdu performed the world’s first genetic editing trial on humans, in an attempt to find a cure for lung cancer using this technique.
- Genetically Modified babies: In 2019, A Chinese scientist claimed that he helped make the world’s first “genetically edited” babies in whom a gene linked to HIV was removed using the CRISPR technique.
- Treating Diseases:
- Successfully inserted TB-resistant gene in cattle in China.
- Possibility to treat Cancer: early-stage clinical trials are being attempted to use the tool to treat some types of cancer.
- Though in 2016 China began the first human clinical trial to treat an aggressive form of lung cancer by introducing cells that contain genes edited using CRISPR-Cas9, the use of the tool has so far been limited to curing genetic diseases in animal models.
- DNA for data Storage: First gene-sequencing is done; Then Gene editing using CRISPR. But the copying speed is low. A short movie has been successfully stored using this.
- Data retention: 100 years; Electronic device: 10 years.
- Power usage is minimal & Data density: is very high.
- Agriculture: It is being tried out in agriculture primarily to increase plant yield, quality, disease resistance, herbicide resistance and domestication of wild species. This can create a large number of crop varieties with improved agronomic performance; it has also brought sweeping changes to breeding technologies.
- Artificial meat.
Other Gene editing Mechanisms:
- ZFNs (zinc-finger nucleases): The DNA-binding part of ZFNs is made of zinc-finger proteins, which each bind to about three DNA bases.
- The nuclease part of ZFNs is normally a FokI nuclease, which cuts the DNA.
- TALENs (Transcription activator-like effector nucleases):
- The DNA-binding domain of TALENs is made of transcription activator-like effector (TALE) domains.
- Like ZFNs, the nuclease part of TALENs is normally a FokI nuclease.
| Nobel Prize in Chemistry for Solving the Protein Folding Puzzle |
| Proteins are formed from only 20 types of amino acids which can be arranged into countless arrangement patterns. However, they always fold in the same arrangement every time and act as enzymes etc very precisely.
Knowing in what shapes the proteins would fold is very difficult to understand for structural biologists. A whole generation of biologists hBiotechnology upscave taken several decades to make a database of the shapes of proteins. However, how the protein would exactly fold is not known. The 2024 Nobel Prize in Chemistry was awarded to David Baker, Demis Hassabis, and John Jumper for their work on solving the protein folding puzzle through computational protein design and structure prediction using artificial intelligence methods; This can solve several problems in the future:
|
Gene Therapy:
A process of introduction, removal or change in the content of an individual’s genetic material to treat the disease and a possibility of achieving a long-term cure.
It is classified into 2 types: Germ-line gene therapy and Somatic Cell Gene therapy.
Germ-line gene therapy:
Introducing gene-modified cells into the germline, that can be transmitted vertically across generations. It is prohibited in India, due to ethical and social considerations.
Somatic cell gene therapy:
It affects the targeted cells/tissue/organs in the patient and is not passed on to subsequent generations (i.e. not introduced in germline- Ex – skin, muscle etc).
- It includes genome modification as done in CRISPR-related and other technologies.
- It is legal in India.
It has two categories:
- Ex vivo– cells obtained from an individual are genetically modified/corrected outside the body followed by transplantation into the same or a different individual.
- In vivo– the gene of interest is delivered directly to target cells/ tissues/organs (like liver, pancreas, muscle, heart etc.) in the patients. Gene delivery can be carried out by viral or non-viral vector systems.
Possibilities:
Around 70 million Indians are estimated to suffer from inherited genetic diseases.
- These include blood disorders such as haemophilia, thalassemia, sickle-cell anaemia etc.
- These can be treated with Gene Therapy.
Gene Therapy Product (GTP):
A biological entity, having the required gene, could introduce modifications in the genome for therapeutic benefit. It works by repairing, replacing or deactivating dysfunctional disease-causing genes aiming to restore normal function.
GTPs include:
- Recombinant viral vectors: For example, adenovirus or retrovirus is attuned and combined with the genes of a new virus(say coronavirus) – This acts as a vaccine: For example, COVAXIN
- Non-viral vectors: naked DNA transfection
- Microbial/bacterial vectors (Salmonella, E. coli): recombinant bacteria-derived vehicles
- Modifications resulting from the use of CRISPR and other similar technologies.
- Ex vivo genetically modified cells: gene-modified/ augmented stem cells, iPS (induced pluripotent stem) cells, CAR-T cells etc.
- Soluble/particulate/emulsion/Nano-based interventions containing any form of genetic material/ nucleic acid for clinical gene therapy.
- DNA vaccines where the final product is nucleic acid and is administered for vaccination/ therapy.
Bio-Manufacturing and Bio-foundry
Bio-manufacturing refers to the use of biological systems such as microbes, enzymes, or cell cultures to produce commercially valuable products like biofuels, pharmaceuticals, biodegradable plastics, industrial enzymes, and food ingredients.
- It represents a shift from traditional chemical manufacturing to more sustainable and eco-friendly biological processes.
- Focus on Regenerative principles:e. restoring, renewing, and revitalizing natural systems. Unlike sustainable practices that aim to minimize harm, regenerative approaches actively improve environmental health, biodiversity, and social equity.
- It aligns with our aim of Atmanirbhar Bharat (self-reliant India) and a transition towards a green, circular, and knowledge-driven economy.
India, with its rich biodiversity, strong pharmaceutical base, and skilled biotechnology workforce, has immense potential in bio-manufacturing. Recognizing this, the Indian government has launched several initiatives to promote bio-foundries and sustainable industrial biotechnology.
Bio-Manufacturing Initiatives in India
- National Mission on Bio-Economy: The government aims to promote bio-based solutions, targeting sectors such as health, agriculture, energy, and environment.
Bio-manufacturing plays a central role in this mission by enabling low-carbon and resource-efficient production. - Biotechnology Industry Research Assistance Council (BIRAC): BIRAC, under DBT, supports start-ups and MSMEs in bio-manufacturing through funding schemes like BioNEST, SPARSH, and BIG (Biotechnology Ignition Grant). These programs help incubate innovations in bio-based production systems, including fermentation technologies, microbial engineering, and biosensors.
- India BioEconomy Report 2022: According to this report, India’s bioeconomy reached $80 billion in 2021 and is projected to grow to $300 billion by 2030, driven by bio-manufacturing, synthetic biology, and green alternatives to petrochemicals.
- Global Biofoundry Alliance: India has expressed interest in joining this international network, which facilitates collaboration, standardization, and knowledge-sharing across national biofoundries.
Bio-foundries
Bio-foundries are integrated facilities that automate and accelerate the design-build-test-learn (DBTL) cycle of biological engineering.
They play a crucial role in synthetic biology by enabling high-throughput production and prototyping of biological systems for bio-manufacturing.
Department of Biotechnology (DBT) Initiatives:
The DBT has supported the creation of bio-foundry platforms in institutions like the Institute of Life Sciences (Bhubaneswar) and the National Institute of Plant Genome Research (NIPGR). These platforms integrate synthetic biology tools with automation, AI, and machine learning to enable rapid design and testing of bio-based products.
India is positioning itself as a global hub for sustainable bio-manufacturing through investment in bio-foundries, policy support, and innovation ecosystems.
Rules & Regulations:
- As per the New Drugs and Clinical Trial Rules (2019), the GTPs fall under ‘new drug’ and shall always be deemed to be ‘new drug’.
- National Guidelines for Gene Therapy-Product (GTP) Development and Clinical Trials, 2019 by ICMR.
- Applicability: The guidelines apply to all stakeholders in the field of gene therapy including researchers, clinicians, regulatory committees, industry, patient support groups etc.
- General Principles: Clinical trials on human participants involving GTPs must safeguard human rights, safety and dignity. Various principles like the Principle of Essentiality, Voluntariness, Non-exploitation, Risk Minimization etc. need to be followed.
- Mechanism for Review and Oversight:
- Gene Therapy Advisory and Evaluation Committee (GTAEC)– an independent body with experts from diverse areas of biomedical research, government agencies and other stakeholders.
- It is mandatory for all institutions and entities engaged in the development of GTPs to establish an Institutional Bio-safety Committee (IBSC).
- Research involving the development of new GTPs needs to obtain approvals from the IBSC and Ethics Committee (EC). Biological material from humans can be procured only from clinics/hospitals that have an Ethics Committee.
- All clinical trials are mandated to be registered with the Clinical Trials Registry-India (CTRI). It is an online public record system for registration of clinical trials being conducted in India.
- Responsibilities of various stakeholders
- Investigators should treat the biological material with utmost respect and adequate care to avoid its misuse.
- Storage and disposal of the GTPs or their components should be as per the Regulations and Guidelines on Bio-safety of Recombinant DNA Research and Bio-containment 2017.
- Any GTP of foreign origin or its modified variants that will be first in human use is not permissible for direct first in human trials in India.
- Investigators should demonstrate respect for the autonomy and privacy of patients.
- Good Manufacturing Practice (GMP) Guidelines:
- It includes Personnel Training and the establishment of quality control processes.
- Waste materials and by-products of the GTP manufacturing process must be securely decontaminated and transported as per appropriate biohazard disposal protocol.
DNA Technologies (Use and Application) Regulation Bill, 2019:
Passed in Lok Sabha.
It Expands the application of DNA-based forensic technologies to support and strengthen the justice delivery system of the country. To use DNA-based utilities for solving crimes, for identifying missing persons etc.
- Consent: Mandatory to all the citizens.
- Exception: Criminal matters involving ‘suspects’ with a punishment of more than 7 years.
- Cases in which Profiling is possible: matters listed in the schedule of the bill.
- Crimes covered under IPC.
- Profiling in civil matters: A scheduled list of civil matters only.
- Problem: consent requirements are not specified for such matters.
- Regulation:
- National and Regional DNA Data Banks: Each data must have the following indices:
- Crime scene index, Suspects‘ index, Offenders‘ index, Missing persons’ index, Unknown deceased persons‘ index.
- DNA profiles can be removed based on a court order, in two cases
- Of an under-trial.
- Or if a police report is filed.
- Missing persons’ profiles can be removed on a written request only.
- DNA profiles can be removed based on a court order, in two cases
- Data must remain protected from misuse or abuse in terms of privacy rights.
- Crime scene index, Suspects‘ index, Offenders‘ index, Missing persons’ index, Unknown deceased persons‘ index.
- DNA Regulatory Board: to accredit the established identity of an individual.
- Mandatory accreditation and regulation of DNA labs.
- For assurance that DNA tests are reliable.
- National and Regional DNA Data Banks: Each data must have the following indices:
Guidelines on nano-Pharmaceuticals
Draft guidelines for evaluation of nano-pharmaceuticals by the Department of Biotechnology under M/o S&T
There are no uniform internationally acceptable guidelines for nano-pharmaceuticals. However, the main challenges faced by regulatory institutions in India include regulatory capacity, information asymmetry, Inter-agency coordination, overlapping roles and mandates etc.
Salient features of the Draft guidelines:
- Aim: to ensure the quality, safety and efficacy as well as encourage the commercialization of nanotechnology-based innovation with a high benefit and low-risk ratio.
- Definition: nano–pharmaceuticals: a pharmaceutical preparation containing nanomaterials (size scale range of 1 to 100nm) intended for internal or external application on the body for therapeutics, diagnostics and any health benefit.
- It also includes preparations with a particle size is >100nm and <1000 nm as nano pharmaceuticals under certain circumstances.
- Categories nano pharmaceuticals:
- According to the degradability of nanomaterial:
- Biodegradable nanoparticles have been used frequently as drug delivery vehicles due to their improved bioavailability, better encapsulation, control release and reduction of toxic potential. Examples: albumin, chitosan, gelatin, polycaprolactone etc.
- Nonbiodegradable nanoparticles are relatively less used in pharmaceutical products (though these systems are more commonly used in cosmeceuticals). Almost all non-biodegradable nanoparticles have the potential to have toxic effects. Examples: titanium oxide, iron oxide, and metals such as gold, silver, platinum, etc.
- According to the nature of nanomaterial:
- Organic Nanoparticles: are nanomaterials or nanoparticles composed of organic compounds like lipids, proteins, and carbohydrates. They have been primarily developed for drug delivery to reduce or overcome the risk of toxicity.
- Inorganic Nanoparticles: are more stable than organic nanostructures. They are easier to prepare with a defined size and a very narrow size distribution. However, most of the inorganic nanoparticles may not be biodegradable.
- Multicomponent nanoparticles are nanoparticles composed of two or more different materials.
- According to the nanoform of the ingredient:
- A nanocarrier is a nanomaterial being used as a transport module for another substance like a drug.
- Some of the conventional/traditional drugs may be converted into nanocrystals, thereby increasing their potential for improved dissolution and bioavailability.
- According to the approval status of the drug and nanomaterial.
- According to the degradability of nanomaterial:
It mandates that the stability testing of nanopharmaceuticals should be done according to the general requirements specified in the Drugs and Cosmetics Rules, 1945.